 The method we use for measuring the exchanges of dissolved materials between the water column and sediments (and vice versa) in shallow estuarine systems involves extracting an intact core of bottom sediments, transferring the sediment core to a plexiglass microcosm, then incubating the microcosm and inferring flux from changes in dissolved material concentrations during a 3-4 hour incubation period. We refer to the general method as the SONE technique (Sediment Oxygen and Nutrient Exchanges). Recently we have used an abbreviated version of our standard SONE technique wherein we use a single core (rather than replicates) and we do not use a water blank as we have found that in virtually all cases the water blank correction was very small. Previous studies had shown that variation among replicate cores from a single location was small compared to variation among different sites. In order to make a more accurate assessment of sediment-water exchanges for entire estuaries, additional stations were added using single rather than the traditional three replicate cores.
The method we use for measuring the exchanges of dissolved materials between the water column and sediments (and vice versa) in shallow estuarine systems involves extracting an intact core of bottom sediments, transferring the sediment core to a plexiglass microcosm, then incubating the microcosm and inferring flux from changes in dissolved material concentrations during a 3-4 hour incubation period. We refer to the general method as the SONE technique (Sediment Oxygen and Nutrient Exchanges). Recently we have used an abbreviated version of our standard SONE technique wherein we use a single core (rather than replicates) and we do not use a water blank as we have found that in virtually all cases the water blank correction was very small. Previous studies had shown that variation among replicate cores from a single location was small compared to variation among different sites. In order to make a more accurate assessment of sediment-water exchanges for entire estuaries, additional stations were added using single rather than the traditional three replicate cores.
 At each site an intact sediment core is collected using a modified Bouma box corer (right). The core is then transferred to a Plexiglass cylinder (d = 15 cm, h = 30 cm) and inspected for disturbances from large macrofauna or cracks in the sediment surface. If the core is satisfactory, it is fitted with an O-ring sealed top containing various sampling ports and a gasket sealed bottom (below). The core is then placed in a darkened, temperature controlled holding tank where the overlying water in the sediment microcosm is slowly replaced by fresh bottom water to ensure that water quality conditions in the microcosm closely approximate in situ conditions. Once the overlying water has been replaced the microcosm is ready for incubation and flux measurements. Cores are placed in a darkened, temperature controlled bath to maintain ambient water temperature and the overlying water is gently circulated without disturbing the sediment surface layer using stirring mechanisms attached to dissolved oxygen sensors (below right). Oxygen concentrations are recorded and overlying water samples are extracted from each core every 60 minutes during the three hour incubation period.
At each site an intact sediment core is collected using a modified Bouma box corer (right). The core is then transferred to a Plexiglass cylinder (d = 15 cm, h = 30 cm) and inspected for disturbances from large macrofauna or cracks in the sediment surface. If the core is satisfactory, it is fitted with an O-ring sealed top containing various sampling ports and a gasket sealed bottom (below). The core is then placed in a darkened, temperature controlled holding tank where the overlying water in the sediment microcosm is slowly replaced by fresh bottom water to ensure that water quality conditions in the microcosm closely approximate in situ conditions. Once the overlying water has been replaced the microcosm is ready for incubation and flux measurements. Cores are placed in a darkened, temperature controlled bath to maintain ambient water temperature and the overlying water is gently circulated without disturbing the sediment surface layer using stirring mechanisms attached to dissolved oxygen sensors (below right). Oxygen concentrations are recorded and overlying water samples are extracted from each core every 60 minutes during the three hour incubation period.
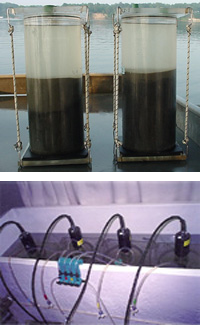
 Water volume is maintained by simultaneous addition of ambient bottom water to the core while the water sample is extracted (below left). Water samples are filtered and immediately frozen for later analysis of dissolved ammonium (NH4+), nitrite (NO2-), nitrite plus nitrate (NO2- + NO3-) and phosphorous (PO4-3). Oxygen and nutrient fluxes are estimated by calculating the rate of change in concentration during the incubation period and converting the volumetric rate to a flux using the volume: area ratio of each core. At each SONE station, water column profiles of temperature, salinity and dissolved oxygen are measured at two meter intervals from the surface to the bottom and surface water turbidity is measured using a Secchi disk (right). We rarely get a secchi disk measurement that exceeds 2 m in the turbid waters of the Bay and tributary rivers.
Water volume is maintained by simultaneous addition of ambient bottom water to the core while the water sample is extracted (below left). Water samples are filtered and immediately frozen for later analysis of dissolved ammonium (NH4+), nitrite (NO2-), nitrite plus nitrate (NO2- + NO3-) and phosphorous (PO4-3). Oxygen and nutrient fluxes are estimated by calculating the rate of change in concentration during the incubation period and converting the volumetric rate to a flux using the volume: area ratio of each core. At each SONE station, water column profiles of temperature, salinity and dissolved oxygen are measured at two meter intervals from the surface to the bottom and surface water turbidity is measured using a Secchi disk (right). We rarely get a secchi disk measurement that exceeds 2 m in the turbid waters of the Bay and tributary rivers.
Near-bottom (approximately ½ m above the bottom) water samples are collected using a high volume submersible pump system. Samples are filtered and immediately frozen for later analysis of the same dissolved nutrients as detailed for the SONE flux measurements. Sediments at each station are characterized by taking smaller (d = 6.5 cm, h = 29 cm) intact sediment cores and measuring the oxidation-reduction (redox) potential (Eh) of the overlying and sediment porewater. Additionally, surficial (surface to 1 cm depth) sediments are sampled for total and active chlorophyll-a, particulate carbon (PC), nitrogen (PN) and phosphorus (PP). Combining all these SONE measurements and variables allows us to examine exchanges and processes occurring between the water column and sediments.
